
Owensby, C.E., R.M. Cochran, and L.M. Auen. 1996. Effects of Elevated Carbon Dioxide on Forage Quality for Ruminants. In Carbon Dioxide, Populations, and Communities. Koerner, C., and F. Bazzaz, eds. Physiologic Ecology Series. Academic Press pp. 363-371. PDF
Introduction
Carbon dioxide (CO2) levels in our atmosphere have increased by 30% over the past 200 years, with much of that increase coming during the past 100 years (Boden et al. 1990). Expectations are that by the middle of the 21st Century atmospheric CO2 will be double current levels. Much conjecture has occurred as to the impact of that increase in atmospheric CO2, primarily centering around climate change, but with a recent concerted effort on the effects of elevated CO2 on terrestrial ecosystem processes. While there have been several research reports concerning the effects of elevated CO2 on insect diet effects (Butler et al. 1986; Lincoln et al. 1986; Fajer 1989; Fajer et al. 1989), there has been relatively little work on ruminant responses. Worldwide, natural ecosystems provide the majority of food resources for ruminants (Semple 1970), with rangelands supplying 95% of the food needs for wild ruminants (Holochek et al. 1989). Therefore, the potential impact of CO2 enrichment on forage quality and dietary conversion efficiencies is of paramount importance. Because nutrient resources in natural systems are fixed to a great degree, the impact of increased carbon fixation with elevated CO2 may lead to dietary deficiencies of essential nutrients for herbivores (Owensby et al. 1993a,b). Although C:N ratios of different plant species have been variable with CO2 enrichment, the prevalent response, when productivity has been increased by CO2 enrichment, has been an increase in the C:N ratio (Newton 1991). Lower nitrogen concentration in forages results in reduced digestibility and conversion efficiency of ingested forage to ruminant growth or reproduction (Huston and Pinchak 1991). Because ruminant digestion is microbial (Hume and Warner 1980), reduced forage quality would lower both the amount of forage digested and the rate of digestion (Huston and Pinchak 1991). Ruminants retain forages in the rumen for extended periods which allows efficient nutrient extraction (Bell 1971).
Based on reported changes in leaf chemistry and insect responses to forages grown under elevated CO2, we present the following hypotheses:Below, we review ruminant digestion processes, the impact of forage quality on forage intake, digestibility, animal productivity, the impact of elevated CO2 on forage quality, and present the results of research on tallgrass prairie.
Ruminant Digestion
Ruminants (cattle, sheep, goats, etc.) have evolved a capacious pre-gastric fermentation structure with four compartments (Fig. 1) where a symbiotic relationship exists with microbes that have an ability to break down complex structural polysaccharides (cellulose and hemicellulose) to compounds that can be absorbed by the animal. That adaptation allows for use of forages produced on the vast rangeland acreage throughout the world. Half the meat and almost all of the milk are produced by ruminant. The fermentation of forage occurs primarily in the reticulo-rumen portion of the gastro-intestinal tract of ruminants. From the reticulo-rumen part of the stomach, food passes into the omasum, whose function is not fully understood, but some absorption of fermentation products occurs there, and then into the abomasum which is similar to the simple stomach of monogastric animals. The rumen develops rapidly and at maturity represents 80% of the stomach capacity (Campbell and Lasley, 1969). The entire stomach occupies three-fourths of the abdominal cavity (Church, 1969). Ruminants only chew the forage enough to mix it with saliva and to form a bolus. Further chewing occurs when the animal regurgitates the bolus during rumination. In order to reduce the particle size of the ingesta, ruminants regurgitate it, swallow regurgitated fluids, remasticate the solids accompanied by re-insalivation, and reswallow the bolus. Rumination has an impact on the digestibility of forages, since rumination increases digestibility by reducing particle size which, in turn, affects average time feed remains in the rumen.
Figure 1.
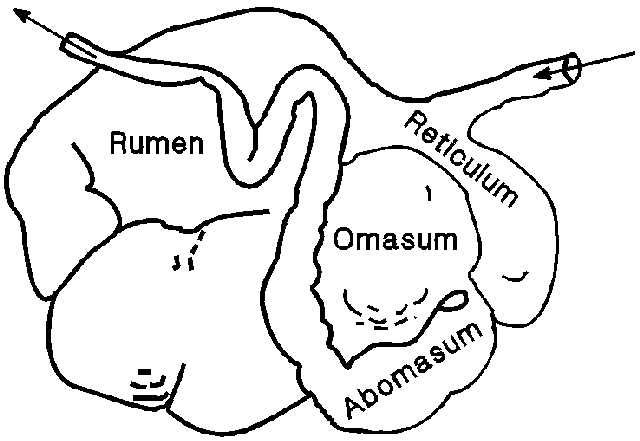
Ruminant digestion is a complex interaction among the diet, the microbial population, and the animal. Within the rumen, flow characteristics can be partitioned into a liquid phase, particles large enough to pass from the rumen and particles that are trapped in the rumen because they are too large to pass through the omasal orifice. The ingesta is in fairly large pieces and floats on top of the rumen fluid. Those larger pieces are involved in rumination and are broken down into smaller pieces. After exposure to the microbial population and rumination which reduce particle size, the ingesta passes into the remainder of the digestive tract. The rumen microbes break down almost all soluble carbohydrates in the forage and degrade 40-80% of the ingested protein. The products of rumen fermentation are volatile fatty acids (acetic, propionic, valeric, and butyric), lactic acid, carbon dioxide, methane, microbial protein, and microbial polysaccharides. The volatile fatty acids are absorbed through the rumen wall into the blood stream for use in metabolism. Lactic acid, microbial protein, and microbial polysaccharides are passed into the remainder of the digestive tract where further digestion and absorption occurs. Methane and carbon dioxide are expelled orally by a process called eructation.
The effect of the ruminant digestive system is to hold the structural components of the ingesta in the rumen for a relatively long period. Bell (1971) stated that the ruminant digestion strategy is to maximize efficiency of the use of protein at the expense of the superabundant energy supply. When cell wall constituents (complex structural polysaccharides) are high in the diet, the ingesta is held longer in the rumen to insure protein extraction. Retention time in the rumen for cattle for most forages is 50-60 hours. Herbaceous dicots generally have lower retention times than grasses. The rate of passage of ingesta through the gastro-intestinal tract increases with low fiber, low lignin diets. Intake of roughages by ruminants is regulated by rumen capacity. The more quickly the rumen empties the greater intake will be. Digestibility of forages determines rate of passage. The more digestible a forage is (low cell wall content, low fiber and lignin content), the greater the rate of passage, with the result being increased intake. The very nature of ruminant digestion makes it impossible for the ruminant to compensate for a lower quality diet by consuming more forage.
Another consequence of global climate change may be an increased air temperature. d Higher daytime air temperature also will reduce forage intake. Dwyer (1961) reported a negative linear relationship between grazing time and average daytime temperature and indicated that there was not an increase in nighttime grazing to compensate for reduced daytime grazing.
Nutrient deficiencies, such as low protein in the forage, will reduce digestibility and therefore rate of passage of forages through the rumen due to lowered microbial activity. Microbes need high amounts of protein to sustain fermentation.
The major impact of lowered forage quality is a reduced intake and consequently lowered productivity. Secondary productivities (rates of energy storage at different trophic levels) in an ecosystem are described by the following diagram:

In ruminants, as the nitrogen concentration of the diet is reduced and fiber component concentrations are increased, the amount of the ingesta assimilated is reduced and egesta is increased. That material is fed directly into the decomposer food chain. Any ecosystem perturbation that reduces forage quality will reduce the assimilation component from which productivity as growth and/or reproduction is derived.
Impact of Elevated CO2 on Forage Quality
Plants exposed to elevated CO2 have consistently had reduced tissue N concentration (Bazzaz 1990; Newton 1991). The reduced N concentration has developed very early in vegetative growth and has persisted to maturity, particularly with C3 species. Conventional wisdom implicated nitrogen dilution with increased carbon acquisition (Field et al. 1992), thereby increasing nitrogen use efficiency (NUE). Numerous studies have shown reductions in N concentration for C3 and C4 species under elevated CO2 across a wide range of nitrogen availabilities (Hocking and Meyer 1985; Larigauderie et al. 1988; Coleman et al. 1991). However, reduced N concentration has been reported in natural ecosystems for species with no increased biomass production under elevated CO2 (Curtis et al. 1989b; Owensby et al. 1993b). The cause may be due to reductions in enzymes associated with photosynthesis (Bowes 1991; Long 1991; Van Oosteen et al. 1992; Wong 1979). Sage et al. (1987) estimated that RuBP and PEPC carboxylases in a C4 and RuBP in a C3 represented as much as 25% of the total leaf N, which could explain decreased N concentration in biomass under elevated CO2. Another possible explanation for a portion of the reduced N concentration of leaves exposed to elevated CO2 may lie in reduced chlorophyll content. Cave et al. (1981) found reduced chlorophyll in Trifolium subterraneum leaves with CO2 enrichment.
Further reductions in forage quality may come from morphologic changes associated with elevated CO2. Thomas and Harvey (1983) reported that leaves of plants under elevated CO2 can have more waxes and extra layers of epidermal cells which may further reduce forage quality for ruminants. Cuticle development appears to be a major deterrent to ruminal digestion. Brazle et al. (1979) and Cummins and Dobson (1972) reported that forage cuticle reduced microbial degradation of ingested forages.
Impact of Elevated CO2 on Cattle Production in Tallgrass Prairie
We collected diet samples from ambient and elevated CO2 (2X ambient) plots using three esophageally-fistulated sheep at 2-week intervals throughout the growing season in 1989 [see Owensby et al. (1993a) for CO2 enrichment procedures] by grazing one half of 4.5 m dia. plots. The diet samples were frozen immediately, freeze-dried, and acid detergent fiber (ADF) (Van Soest 1967) and N concentration (Linder and Harley 1942; Technicon 1977) determined.
Forage quality tests individually indicate relative nutritive value among treatments, but fail to integrate the impact of a treatment across different measured parameters. For example, a reduction in nitrogen concentration or an increased fiber concentration indicate a reduction in forage quality, but they do not indicate the impact on the efficiency of utilization of the energy in the diet or the impact on ruminant production. We used the ADF and N values from the ambient and elevated CO2 diet samples to estimate the growth response of yearling steers grazing tallgrass prairie.
Chemical composition affects the energetic value of plant materials when used as feeds for livestock (Blaxter, 1962). Therefore, we estimated the magnitude of the impact on livestock gain that could result from the changes in chemical composition observed in response to enhanced CO2 concentration. Weight gain projections were calculated for beef cattle that were assumed to be between 12 and 24 months of age and experiencing relatively rapid growth while grazing tallgrass prairie during the late spring and summer periods.
The initial step in the simulation process involved estimation of organic matter digestion (OMD) from the ADF concentration (Minson, 1982). Accuracy of OMD predictions using this approach has been quite good when compared with OMD values determined directly in cattle consuming tallgrass-prairie forage (Sunvold and Cochran, 1992). Subsequently, digestible energy (DE) concentration was estimated from the OMD concentration and then the metabolizable energy (ME) concentration was predicted from the DE concentration (Minson, 1990). The efficiency of ME use for maintenance (km) and the efficiency of ME use for production (i.e., gain; kf+p) were estimated using the associated protein values and ME concentrations (expressed as a percentage of gross energy) as described by Blaxter (1989). Metabolizable energy values and their associated efficiencies were then used to estimate the gain that might be realized by a rapidly growing, yearling steer consuming a given amount of forage. Although weight would obviously be changing over the course of the grazing period, to simply the simulations an average weight of 250 kg was used in all calculations and forage intake was assumed to be the same for cattle consuming forage from CO2-enriched and ambient CO2 environments (intake would likely be lower for cattle consuming forage produced in an elevated CO2 environment). Amount of forage intake was assumed to decrease as season progressed based on measurements from previous studies at our location. The amount of net energy needed for maintenance was estimated by dividing the km into the sum of an estimate of fasting heat production (FHP) and activity (Agricultural Research Council, 1980). Once maintenance was accounted for, we estimated the amount of gain that could be supported from the remaining ME. This was determined by multiplying the ME available for gain by the kf+p . Finally, the calorific values of weight gain presented by Blaxter (1962) were used to convert megacalories of gross energy in the gain into weight gain values.
Estimated gain for steers consuming forage produced under elevated CO2 in 1989 was lower than that produced under ambient CO2 summed over the 150-day growth period (2X CO2 - 80.6 kg; 1X CO2 - 99.6 kg), with the greatest reduction in gain coming in the early season (Fig.2).
Figure 2.

Conclusions
Since N and fiber concentrations in the diet of ruminants impact forage digestibility and utilization efficiency, the reported reduced N and increased fiber concentrations in plants grown under elevated CO2 will likely impact ruminant productivity negatively. Data reporting reduced productivity or increased consumption for insects consuming diets of plants grown under elevated CO2 support that conclusion. Contrary to the results from insect studies, where intake increased as diet quality decreased, ruminant intake declines as forage quality decreases. Therefore, there cannot be a compensatory intake response to maintain productivity levels comparable to current levels. For domestic livestock, diets can be supplemented to compensate for reduced forage quality, but with wild ruminants, or for ruminants in developing countries, diet supplementation is not an option. The result will be reduced growth and reproduction. Further, changes in climate may impact foraging by ruminants. High daytime air temperatures currently reduce total grazing time for cattle with little or no compensatory nighttime grazing.
A future high CO2 world seems destined to reduce individual animal performance. For domestic livestock enterprises, increased stocking rates can occur because of the reduced intake of lower quality forage, and dietary supplementation may be used to maintain current production levels, but that will increase cost of production. Wild ruminant diet quality will be affected, and it is likely that they will have reduced growth and reproduction.
Literature Cited
Agricultural Research Council. 1980. The Nutrient Requirements of Ruminant Livestock. C.A.B. International, Wallingford, U.K. pp. 118
Bazzaz, F.A. 1990. The response of natural ecosystems to the rising global CO2 levels. Ann. Rev. Ecol. Syst. 21:167-196
Blaxter, K.L. 1962. The Energy Metabolism of Ruminants. Hutchinson & Co., Ltd., London. pp. 187, 169
Blaxter, K.L. 1989. Energy Metabolism in Animals and Man. Cambridge University Press, Cambridge, U.K. pp 275
Bowes, G. 1991. Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant, Cell and Environment 14:795-806.
Bell, R.H.V. 1971. A grazing ecosystem in the Serengeti. Sci. Amer. 225:86-93.
Boden, T.A., P. Kanciruk, and M.P. Farrell. 1990. Trends '90: A compendium of data on global climate change. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge TN. 257 pp.
Brazle, F. K., L. H. Harbers, and C. E. Owensby. 1979. Structural inhibitors of big and little bluestem digestion observed by scanning electron microscopy. J. Anim. Sci. 48:1457-1463.
Butler, G.D., B.A. Kimball, and J.R. Murray. 1986. Populations of Bermisia tabaci (Homoptera: Aleyrodae) on cotton grown in open-top chambers enriched with CO2. Environmental Entymology 15:61-63.
Campbell, J. R.,and Lasley, J.F. 1969. The Sciences of Animals That Serve Mankind. McGraw-Hill Book Co. New York. 771p.
Cave, G., L.C. Tolley, and B.R. Strain. 1981. Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiol. Plant. 51:171-174.
Church, D. C. 1969. Digestive Physiology and Nutrition of Ruminants. Volume I. Oregon State University. Corvallis. 316 p.
Coleman, J.S., L. Rochefort, F.A. Bazzaz and F.I. Woodward. 1991. Atmospheric CO2, plant nitrogen status and the susceptibility of plants to an acute increase in temperature. Plant, Cell and Environment 14:667-674.
Cummins, D. G., and J. W. Dobson, Jr. 1972. Digestibility of bloom and bloomless sorghum leaves as determined by a modified in vitro technique. Agron. J. 64:682-683.
Curtis, P.S., B.G. Drake, and D.F. Whigham. 1989. Nitrogen and carbon dynamics in C3 and C4 estuarine marsh plants grown under elevated CO2 in situ. Oecologia 78:297-301.
Dwyer, D. D. 1961. Activities and grazing preferences of cows with calves in northern Osage County, Oklahoma. Okla. Agr. Expt. Sta. Bul. B-558. 61 p.
Fajer, E.D. 1989. The effects of enriched CO2 atmospheres on plant-insect herbivore interactions: growth responses of larvae of the specialist butterfly, Junonia coenia (Lepidoptera: Nymphalidae). Oecologia 81:514-520.
Fajer, E.D., M. D. Bowers, F.A. Bazzaz. 1989. The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243:1198-1200.
Field, B.F., F.S. Chapin III, P.A. Matson, and H.A. Mooney. 1992. Responses of terrestrial ecosystems to the changing atmosphere: A resource-based approach. Annu. Rev. Ecol. Syst. 23:201-235.
Hocking, P.J. and C.P. Meyer. 1985. Responses of noogoora burr (Xanthium occidentale Bertol.) to nitrogen supply and carbon dioxide enrichment. Annals of Botany 55:835-844.
Holochek, J.L., R.D. Peiper, and C.H. Herbel. 1989. Range Management: Principles and Practices. Prentice-Hall, Inc. Englewood Cliffs, NJ. 501 p.
Hume, I.D., and A.C.I. Warner. 1980. Evolution of microbial digestion in mammals. p.665-684. In Y. Ruckebusch and P. Thivend (eds.). Digestive Physiology and Metabolism in Ruminants. AVI. Westport, CT, USA.
Huston, J.E., and W.E. Pinchak. 1991. Range Animal Nutrition. p. 27-64. In: Grazing Management: An Ecological Perspective. R.K. Heitschmidt and J.W. Stuth, eds. Timber Press. Portland OR.
Larigauderie, A., D.W. Hilbert, and W.C. Oechel. 1988. Effect of CO2 enrichment and nitrogen availability on resource acquisition and resource allocation in a grass, Bromus mollis. Oecologia 77:544-549.
Lincoln, D.E., D. Couvet and N. Sionit. 1986. Response of an insect herbivore to host plants grown in carbon dioxide enriched atmospheres. Oecologia 69:556-560.
Linder, R.C., and C.P. Harley. 1942. A rapid method for the determination of nitrogen in plant tissue. Science 96:565-566.
Long, S.P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant, Cell and Environment 14:729-739.
Minson, D.J. 1982. Effect of chemical composition on feed digestibility and metabolizable energy. Nutrition Abstracts and Reviews - Series B 52:592-612.
Minson, D.J. 1990. Forage in Ruminant Nutrition. Academic Press, Inc. San Diego, CA. pp 93-95.
Newton, P.C.D. 1991. Direct effects of increasing carbon dioxide on pasture plants and communities. New Zealand J. Agr. Res. 34:1-24.
Owensby, C.E., P.I. Coyne, J.M. Ham, L.M. Auen, and A.K. Knapp. 1993a. Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated levels of CO2. Ecological Applications 3:644-653.
Owensby, C.E., P.I. Coyne, and L.M. Auen. 1993b. Nitrogen and phosphorus dynamics of a tallgrass prairie ecosystem exposed to elevated carbon dioxide. Plant, Cell, and Environment 16:843-850
Sage, R.F., R.W. Pearcy, and J.R. Seemann. 1987. The nitrogen use efficiency of C3 and C4 plants. Plant Physiol. 85:355-359.
Semple, A. T. 1970. Grassland Improvement. CRC Press. Cleveland. 400 p.
Sunvold, G.D., and R.C. Cochran. 1991. Technical note: Evaluation of acid detergent lignin, alkaline peroxide lignin, acid insoluble ash, and indigestible acid detergent fiber as internal markers for prediction of alfalfa, bromegrass, and prairie hay digestibility by beef steers. J. Anim. Sci. 69:4951-4955.
Technicon Industrial Systems. 1977. Individual/simultaneous determination of nitrogen and/or phosphorus in BD acid digests. Industrial Method NO. 334-74WB+.
Thomas, J.F. and C.N. Harvey. 1983. Leaf anatomy of four species grown under continuous CO2 enrichment. Bot. Gaz. 144:303-309.
van Oosten, J.J., D. Afif, and P. Dizengremel. 1992. Long-term effects of a CO2 enriched atmosphere on enzymes of the primary carbon metabolism of spruce tree. Plant Physiol. Biochem. 30(5):541-547.
Van Soest, P.J. 1967. Development of a comprehensive system of feed analyses and its application to forages. J. Anim. Sci. 26:119-128.
Wong, S.C. 1979. Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen nutrition and photosynthetic capacity in C3 and C4 plants. Oecologia 44:68-74.